RARITAN, N.J., April 28, 2015 /PRNewswire-HISPANIC PR WIRE/ — Janssen Pharmaceuticals, Inc., the manufacturer of INVOKANA® (canagliflozin) for the treatment of type 2 diabetes, today announced the launch of a multifaceted initiative to address the culturally-unique needs of Hispanic adults with type 2 diabetes and their healthcare professionals. The initiative is designed to help improve type 2 diabetes care, education and management in the Hispanic community through training and education programs, INVOKANA® patient access services and support resources like INVOKANA® CarePath™, and tools to help healthcare professionals overcome cultural and linguistic barriers to providing more effective care for Hispanic patients.
Experience the interactive Multimedia News Release here http://www.multivu.com/players/English/7504451-janssen-hispanic-diabetes-initiative/
“The growth of the U.S. Hispanic population and its status as our nation’s largest minority represents unique opportunities and also challenges in ensuring a healthy population,” said Elena Rios, M.D., president of the National Hispanic Medical Association. “Diabetes is an increasing problem in our community. Hispanic patients perceive and manage diabetes quite differently than other populations, which makes culturally-relevant information, care and practices essential, and highlights why this Janssen initiative is so important.”
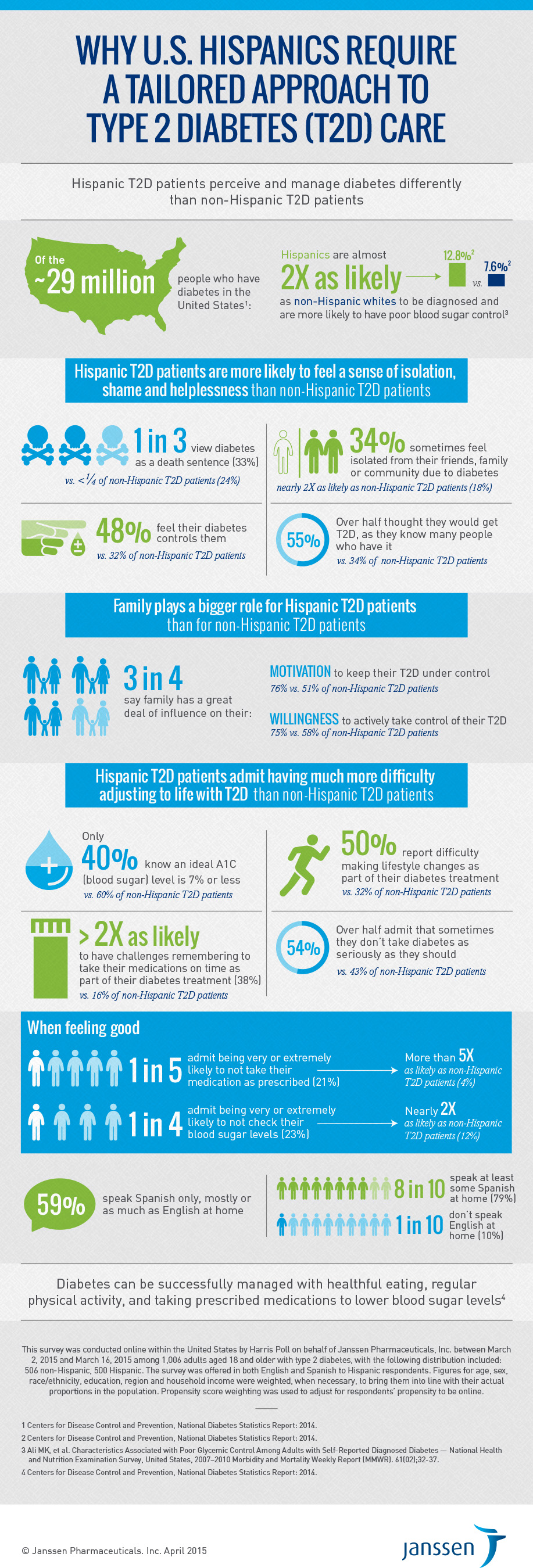
Results of a recent U.S. Hispanic insights survey bring to life these cultural differences. The survey showed that Hispanics with type 2 diabetes are nearly twice as likely as non-Hispanic patients to feel isolated because of diabetes (34% vs. 18%), are more motivated by family to manage the disease (76% vs. 51%), and are much more likely to have difficulty adjusting to life with type 2 diabetes (50% vs. 32%). In addition, fewer Hispanic type 2 diabetes patients are aware of the American Diabetes Association’s recommended target A1C (blood sugar) level of 7 percent or less compared with non-Hispanic type 2 diabetes patients (40% vs. 60%), and are more than five times as likely to not take their medication as prescribed when feeling good (21% vs. 4%) than non-Hispanic type 2 diabetes patients.
Diabetes is more prevalent in the Hispanic population than in the non-Hispanic white population in the United States: Of the approximately 54 million Hispanics who are living in the United States,1 12.8 percent have diagnosed diabetes compared to 7.6 percent of non-Hispanic whites.2 Blood sugar control is a particular challenge. While nearly half of all adults with type 2 diabetes do not achieve recommended levels of blood sugar control, leading a healthy lifestyle through diet and exercise, and taking prescribed medications, can help reduce the risk of serious complications.
“We take seriously our responsibility to address the need for culturally-relevant information about type 2 diabetes and treatment approaches, such as INVOKANA®,” said Nauman Shah, Vice President of Sales and Marketing, Metabolics, Janssen. “Our goal is that Hispanic patients and their healthcare professionals can work together to determine appropriate diabetes care regimens.”
INVOKANA® (canagliflozin) is a once-daily pill used along with diet and exercise to lower blood glucose in adults with type 2 diabetes. It is the first in a new class of medications called sodium glucose cotransporter 2 (SGLT2) inhibitors available in the United States.
Through this initiative, Janssen will introduce tailored INVOKANA® digital platforms and patient support resources that are inspired by the important role of family in the Hispanic population, address gaps in understanding key diabetes health measures, and provide practical lifestyle management resources such as healthy eating and exercise plans that are relevant to Hispanic patients.
Resources for healthcare professionals include patient profiles showing key differences among Hispanic type 2 diabetes patients with varied cultural backgrounds, and a first-of-its-kind patient decision aid to help physicians tailor treatment approaches based on individual patient preferences. Expert steering committees of healthcare professionals with extensive experience in providing care to Hispanic and Latino Americans guided the development of these and other resources.
“With this initiative, we hope to inspire healthier living among Hispanic type 2 diabetes patients and provide trusted guidance, resources and support that will help this fast-growing community succeed in better managing diabetes,” said Melissa Larrazabal, Product Manager, Hispanic Program, Janssen.
Visit www.Invokana.com/es for more information and to access online educational resources that offer culturally-relevant, Spanish-language type 2 diabetes management information for patients and healthcare professionals.
For additional findings from the Hispanic insights survey, click here.
About Type 2 Diabetes
Of the approximately 29 million people who have diabetes in the United States, 90 to 95 percent of them have type 2 diabetes,5 which is chronic and affects the body’s ability to metabolize sugar (glucose), and is characterized by the inability of pancreatic beta cell function to keep up with the body’s demand for insulin.
Nearly half of adults with type 2 diabetes do not achieve recommended levels of glucose control, and if left uncontrolled, type 2 diabetes can lead to serious complications.3,4,5 Improved glycemic control has been demonstrated to reduce the onset and progression of these complications.5
Insights Survey Design
The Hispanic insights survey was conducted online within the United States by Harris Poll on behalf of Janssen Pharmaceuticals, Inc. between March 2, 2015 and March 16, 2015 among 1,006 adults aged 18 and older with type 2 diabetes, with the following distribution included: 506 non-Hispanic, 500 Hispanic. The survey was offered in both English and Spanish to Hispanic respondents. Figures for age, sex, race/ethnicity, education, region and household income were weighted, when necessary, to bring them into line with their actual proportions in the population. Propensity score weighting was used to adjust for respondents’ propensity to be online.
WHAT IS INVOKANA®?
INVOKANA® is a prescription medicine used along with diet and exercise to lower blood sugar in adults with type 2 diabetes. INVOKANA® is not for people with type 1 diabetes or with diabetic ketoacidosis (increased ketones in blood or urine). It is not known if INVOKANA® is safe and effective in children under 18 years of age.
IMPORTANT SAFETY INFORMATION
INVOKANA® can cause important side effects, including:
- Dehydration (the loss of body water and salt), which may cause you to feel dizzy, faint, lightheaded, or weak, especially when you stand up (orthostatic hypotension). You may be at higher risk of dehydration if you have low blood pressure, take medicines to lower your blood pressure (including diuretics [water pills]), are on a low sodium (salt) diet, have kidney problems, or are 65 years of age or older
- Vaginal yeast infection. Women who take INVOKANA® may get vaginal yeast infections. Symptoms include: vaginal odor, white or yellowish vaginal discharge (discharge may be lumpy or look like cottage cheese), or vaginal itching
- Yeast infection of the penis (balanitis or balanoposthitis). Men who take INVOKANA® may get a yeast infection of the skin around the penis. Symptoms include: redness, itching, or swelling of the penis; rash of the penis; foul-smelling discharge from the penis; or pain in the skin around penis
Talk to your doctor about what to do if you get symptoms of a yeast infection of the vagina or penis.
Do not take INVOKANA® if you:
- are allergic to canagliflozin or any of the ingredients in INVOKANA®. Symptoms of allergic reaction may include: rash; raised red patches on your skin (hives); or swelling of the face, lips, tongue, and throat that may cause difficulty in breathing or swallowing
- have severe kidney problems or are on dialysis
Before you take INVOKANA®, tell your doctor if you have kidney problems, liver problems, are on a low sodium (salt) diet, ever had an allergic reaction to INVOKANA®, or have other medical conditions.
Tell your doctor if you are or plan to become pregnant, are breastfeeding, or plan to breastfeed. It is not known if INVOKANA® will harm your unborn baby. It is also not known if INVOKANA® passes into your breast milk.
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements. Especially tell your doctor if you take diuretics (water pills), rifampin (used to treat or prevent tuberculosis), phenytoin or phenobarbital (used to control seizures), ritonavir (Norvir®, Kaletra®, Lopinavir® – used to treat HIV infection), or digoxin (Lanoxin® – used to treat heart problems).
Possible Side Effects of INVOKANA®
INVOKANA® may cause serious side effects, including: kidney problems, a high amount of potassium in your blood (hyperkalemia), or low blood sugar (hypoglycemia). If you take INVOKANA® with another medicine that can cause low blood sugar, such as a sulfonylurea or insulin, your risk of getting low blood sugar is higher. The dose of your sulfonylurea medicine or insulin may need to be lowered while you take INVOKANA®.
Signs and symptoms of low blood sugar may include: headache, drowsiness, weakness, dizziness, confusion, irritability, hunger, fast heartbeat, sweating, shaking, or feeling jittery.
Serious allergic reaction. If you have any symptoms of a serious allergic reaction, stop taking INVOKANA® and call your doctor right away or go to the nearest hospital emergency room.
The most common side effects of INVOKANA® include: vaginal yeast infections and yeast infections of the penis; urinary tract infection; or changes in urination, including urgent need to urinate more often, in larger amounts, or at night.
Tell your doctor if you have any side effect that bothers you or that does not go away. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Janssen Scientific Affairs, LLC at 1-800-526-7736.
Please see the full Product Information and Medication Guide.
Canagliflozin is licensed from Mitsubishi Tanabe Pharma Corporation.
Trademarks are those of their respective owners.
About Janssen Pharmaceuticals, Inc.
As a member of the Janssen Pharmaceutical Companies, Janssen Pharmaceuticals, Inc. is dedicated to addressing and resolving the major unmet medical needs of our time. Driven by our commitment to patients, healthcare professionals, and caregivers, we strive to develop sustainable and integrated healthcare solutions by working in partnership with all stakeholders on the basis of trust and transparency. Our daily work is guided by meeting goals of excellence in quality, innovation, safety, and efficacy in order to advance patient care.
For more information on Janssen Pharmaceuticals, Inc., visit us at www.janssenpharmaceuticalsinc.com or follow us on Twitter at www.twitter.com/JanssenUS and on YouTube at www.Youtube.com/JanssenUS.
1 Centers for Disease Control and Prevention, “Hispanic or Latino Populations.” Available at: http://www.cdc.gov/minorityhealth/populations/REMP/hispanic.html.
2 Centers for Disease Control and Prevention, National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014. Available at: http://www.cdc.gov//diabetes/pubs/statsreport14/national-diabetes-report-web.pdf
3 Bailey CJ. Renal glucose reabsorption inhibitors to treat diabetes. Trends Pharmacol Sci. 2011;32(2):63-71.
4 Casagrande SS, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care. 2013 Feb 15. Epub ahead of print.
5 World Health Organization, Media Centre, Diabetes, Fact sheet Number 312. Available at: http://www.who.int/mediacentre/factsheets/fs312/en/.